
A. Links:
Ludwig Schneider Letter Mercury-2017 >> ![]()
State Mercury-Added Product Ban & Phase-out Guidance >>
BAN MERCURY THERMOMETERS
House Bill 4599 as enrolled
Public Act 578 of 2002
Second Analysis (10-10-02)
Sponsor: Rep. Jack Minore
House Committee: Commerce
Senate Committee: Natural Resources and Environmental Affairs
THE APPARENT PROBLEM:
According to the federal Environmental Protection Agency,
Mercury is a toxic substance that can harm both humans and wildlife. Many different products, including thermometers, contain mercury. When these products break, the mercury can evaporate, creating a risk of dangerous exposures to mercury vapor in indoor air. Moreover, mercury that volatizes when products break in the home or in the waste disposal system enter the environment and can be deposited in lakes and rivers, where it can be transformed into highly toxic methylmercury. Very small deposits of mercury can do significant damage. One gram of mercury per year is enough to contaminate all the fish in a lake with [a] surface area of 20 acres.
A representative of the Ecology Center in Ann Arbor testified before the House Commerce Committee that “mercury attacks the central nervous system and can cause tremors, impaired vision and hearing, developmental deficits during fetal development, attention deficit, and developmental delays during childhood.” Fetuses and children under six are said to be especially vulnerable. The contamination of lakes and fish by mercury is said to be an important public health problem.
As mentioned, one source of mercury is the mercury thermometer. (Other product sources include batteries, automobile switches, and fluorescent bulbs. Coal-fired electric utilities, municipal waste combusters, and medical waste incinerators are said to be the principal sources of mercury in the air.) Environmentalists say that mercury thermometers are responsible for ten percent of the mercury in the municipal waste stream. Moreover, they say, in one recent year, poison control centers received 18,000 calls from people who had broken a mercury fever thermometer in the home. While health officials say that breaking a thermometer is not likely to threaten an individual’s health, they report that there have been cases of serious illness and even death related to the exposure to mercury from fever thermometers; young children are said to be most susceptible. The EPA has said, “Clearly, thermometers are not the major source of mercury to the environment, but they are a meaningful small source that can be relatively easily reduced”. Environmentally safer and affordable alternatives to mercury fever thermometers are readily available, including digital electronic thermometers.
As a result, a number of states have banned or limited the sale of mercury thermometers, including California, Oregon, Rhode Island, Maine, Maryland, Indiana, Minnesota, and New Hampshire. Some local units of government across the country have issued bans as well, including Ann Arbor. A recent report from the American Academy of Pediatrics called for the end to the use of mercury-containing thermometers, according to an AAP press release. Reportedly, the EPA and the American Hospital Association have signed a memorandum of understanding agreeing to try to eliminate mercury from health care, and some national retail chains have stopped selling mercury thermometers. Legislation has been introduced that would ban the sale (with some exceptions) of mercury thermometers in Michigan.
THE CONTENT OF THE BILL:
The bill would amend Part 172 of the Natural Resources and Environmental Protection Act (NREPA) to prohibit, beginning January 1, 2003, a person from selling, offering to sell, or offering for promotional purposes a mercury thermometer in the state or for use in the state, except in certain specified circumstances.
The bill would permit a mercury thermometer to be sold or offered when its use was required by state or federal statute, regulation, or administrative rule or for pharmaceutical research purposes. The bill would permit the sale or offering of mercury fever thermometers by prescription. A manufacturer of mercury fever thermometers would be required to supply with each thermometer sold by prescription clear instructions on the careful handling of the thermometer to avoid breakage and on proper cleanup should the thermometer break.
The bill’s provisions would be enforced by the Department of Environmental Quality. A violation would be a misdemeanor punishable by imprisonment for not more than 60 days or a fine of not more than $1,000, or both, plus the costs of prosecution.
The term “mercury thermometer” would be defined to mean a product or component, other than a dry cell battery, of a product used for measuring temperature that contains mercury or a mercury compound intentionally added to the product or component. A “mercury fever thermometer” would be defined as a mercury thermometer used for measuring body temperature.
MCL 324.17201
BACKGROUND INFORMATION:
The web site of the federal Environmental Protection Agency contains a great deal of information on mercury generally and on mercury thermometers. The address is www.epa.gov.
FISCAL IMPLICATIONS:
The House Fiscal Agency reports that the bill would result in increased costs to the Department of Environmental Quality. The actual budget impact would depend on the number of inspectors added. The $1,000 fine for violations would go to local government to support public libraries. (HFA fiscal note dated 2-11-02)
ARGUMENTS:
For:
Proponents say that the bill represents a small but significant step in reducing the threat to the environment and public health from mercury. Michigan would join the list of states (and local jurisdictions) prohibiting or limiting the sale of mercury thermometers. While the obvious benefit from banning mercury thermometers is to individual households, there will also be some benefit to society at large. The federal EPA notes that when mercury thermometers break or are disposed of, say in incinerators, mercury enters the environment. The agency has said that “combustion of various mercury-containing products in municipal solid waste is the second largest source of mercury to the environment [and] the fourth largest source . . . is combustion of medical wastes. These two categories together account for nearly one-third of the mercury released to the atmosphere”. Federal officials say mercury thermometers contribute 17 tons of mercury each year to the municipal solid waste stream. And each year thousands of people break mercury thermometers in their homes. There are a number of suitable alternatives to mercury fever thermometers; indeed, some retailers have already stopped carrying the mercury thermometer. The bill takes a prospective approach; it does not anticipate removing existing mercury thermometers but prohibiting future sales. It should be also noted that the bill allows for special circumstances when such thermometers may be necessary.
Analyst: C. Couch
A. REPLACING MERCURY CONTAINING PRODUCTS AND MERCURY THERMOMETERS IN ASTM STANDARDS
Abstract
At the request of several States, ASTM International has issued a directive to all technical committees to review their standards that reference mercury containing instruments or mercury methods and determine the technical and economic feasibility of replacing them or substituting with non-mercury instruments or methods. A number of States have banned the sale of mercury containing instruments, including ASTM mercury thermometers, making it difficult for users in those States to comply with ASTM standards. In most cases, the instruments in question are the mercury-in-glass thermometers specified in ASTM E1. These thermometers have long been the gold standard temperature measurement devices in many ASTM standards. In this presentation we will review the ASTM directive, including how to communicate the technical committees’ resolutions to ASTM; technical issues in replacing mercury containing devices with other devices, especially the thermometers found in ASTM E1; legal issues involved with this project, including which States have bans and how the bans differ from State to State.
 Most well-constructed thermometers contain an expansion chamber or blister at the extreme top of the capillary. This chamber serves a twofold purpose. One, to accommodate an overflow of mercury when the thermometer is subject to temperatures in excess of its scale range, and two, as a means of rejoining this type of separation, While holding the instrument in a vertical position, slowly heat the bulb until the separated segments and a portion of the main (intact) column enter the chamber. (Great care must be taken to insure that the mercury does not fill more than ½ to ¾ of the chamber volume: otherwise, breakage of the bulb will result). The nitrogen pressure will force a rejoining of the mercury. Still holding the thermometer vertically, examine the column as it cools and retracts to be sure it is intact.
SEPARATION IN THE CONTRACTION CHAMBER
Most well-constructed thermometers contain an expansion chamber or blister at the extreme top of the capillary. This chamber serves a twofold purpose. One, to accommodate an overflow of mercury when the thermometer is subject to temperatures in excess of its scale range, and two, as a means of rejoining this type of separation, While holding the instrument in a vertical position, slowly heat the bulb until the separated segments and a portion of the main (intact) column enter the chamber. (Great care must be taken to insure that the mercury does not fill more than ½ to ¾ of the chamber volume: otherwise, breakage of the bulb will result). The nitrogen pressure will force a rejoining of the mercury. Still holding the thermometer vertically, examine the column as it cools and retracts to be sure it is intact.
SEPARATION IN THE CONTRACTION CHAMBER
 Separation of the mercury in the contraction chamber is an entirely different problem. Many thermometers contain scale ranges that begin well above ambient temperature. These Thermometers have a contraction chamber or enlargement that prevents the mercury from entering the bulb at ambient temperatures. Heavy jarring of the instrument or thermal shock can cause separation in the chamber. If the separated mercury is in the form of a speck or small amount, invert the thermometer and gently tap it against palm of the hand. This will cause a larger separation, adding additional volume and weight to the separation portion. The thermometer should then be righted and only the tip of the bulb should be dipped in ice water or salted ice water. Remove it from water and gently tap the bulb on a desk blotter and allow the separation portion to fall to the bottom of the chamber and re-join the mercury reservoir. Allow thermometer to warm to room temperature and check to be sure all separations have been removed.
SEPARATION IN THE LOWER PORTION OF THE COLUMN
Separation of the mercury in the contraction chamber is an entirely different problem. Many thermometers contain scale ranges that begin well above ambient temperature. These Thermometers have a contraction chamber or enlargement that prevents the mercury from entering the bulb at ambient temperatures. Heavy jarring of the instrument or thermal shock can cause separation in the chamber. If the separated mercury is in the form of a speck or small amount, invert the thermometer and gently tap it against palm of the hand. This will cause a larger separation, adding additional volume and weight to the separation portion. The thermometer should then be righted and only the tip of the bulb should be dipped in ice water or salted ice water. Remove it from water and gently tap the bulb on a desk blotter and allow the separation portion to fall to the bottom of the chamber and re-join the mercury reservoir. Allow thermometer to warm to room temperature and check to be sure all separations have been removed.
SEPARATION IN THE LOWER PORTION OF THE COLUMN
 Separations of this type are less frequent and more difficult to repair. There are many variables and no one explanation will cover all types of thermometer. The general procedure is to subject the bulb only to a temperature sufficient to retract all the mercury into the bulb. A slow return to ambient temperature will return an intact column. Our experience has shown that additional problems can be caused in these procedures and we point out the following cautions. Cautions must be taken where a range is such that at the freezing point of mercury -38.8°C (almost the same in °F) mercury still remains in the capillary. If the thermometer is returned to ambient temperatures rapidly after freezing, there is a chance the bulb will crack. This Breakage is caused by the mercury thawing in the capillary more slowly then the mercury in the bulb, thereby creating an impasse to the expanding mercury. To avoid the breakage, great care must be taken to allow the mercury in the bulb to liquefy at the same rate as the mercury in the capillary. While the thermometer is being allowed to return to ambient temperature, extreme care must be taken to insure that it is not jarred or at the slightest angle while the mercury returns to the capillary. Without these precautions, gas bubbles could develop in the bulb and cause inaccuracies.
In this case, request an RGA to return the thermometer to the manufacturer to reunite and return (at customer expense).
Separations of this type are less frequent and more difficult to repair. There are many variables and no one explanation will cover all types of thermometer. The general procedure is to subject the bulb only to a temperature sufficient to retract all the mercury into the bulb. A slow return to ambient temperature will return an intact column. Our experience has shown that additional problems can be caused in these procedures and we point out the following cautions. Cautions must be taken where a range is such that at the freezing point of mercury -38.8°C (almost the same in °F) mercury still remains in the capillary. If the thermometer is returned to ambient temperatures rapidly after freezing, there is a chance the bulb will crack. This Breakage is caused by the mercury thawing in the capillary more slowly then the mercury in the bulb, thereby creating an impasse to the expanding mercury. To avoid the breakage, great care must be taken to allow the mercury in the bulb to liquefy at the same rate as the mercury in the capillary. While the thermometer is being allowed to return to ambient temperature, extreme care must be taken to insure that it is not jarred or at the slightest angle while the mercury returns to the capillary. Without these precautions, gas bubbles could develop in the bulb and cause inaccuracies.
In this case, request an RGA to return the thermometer to the manufacturer to reunite and return (at customer expense).
 Correction Table for Specific Gravity Hydrometers
Correction Table for Specific Gravity HydrometersA. The most common industrial instrument for measuring liquid density is the hydrometer and the most common of the hydrometers used to measure brine strength is the Salometer.
The Salometer scale directly indicates the percent of saturation of the brine, reading 0º in pure water and 100ºS in fully saturated brine. By definition, the Salometer degree indicates the percent of saturation, i.e. a 70ºS is 70 percent saturated. Therefore, in calibrating Salometer scales or computing brine tables based on this scale, it is
necessary first to establish the percent value of a fully saturated brine as the fundamental unit and then divide this percent into 100 parts. The Salometer degree and the percent salt are thus rigidly tied together by formula:
Degrees S = % salt – brine X 100
% salt – saturated brine
Occasionally a special type of Salometer is used in the canning industry and for testing brine used in quality grading. It is graduated on a scale where 100ºS represents brine containing 25% salt, instead of saturated brine containing 26.395% salt.
Ordinarily, Salometers are scaled for reading at a temperature of 60ºF, but special Salometers are available for the meat packing industry scaled for readings at 38ºF.
Proper procedure reading a salometer:
Select a clean. straight-walled glass cylinder with a diameter at least twice that of the salometer bulb and with sufficient height to allow for complete immersion of the salometer scale. The cylinder should be tall enough to allow the salometer to float at a O°S reading.
Place the cylinder on a level surface and fill with a sufficient volume of brine to raise the liquid level near the top of the cylinder after the salometer is immersed.
Record the brine temperature. lf significantly different than 60°F, a temperature correction to the reading will be necessary (see chart).
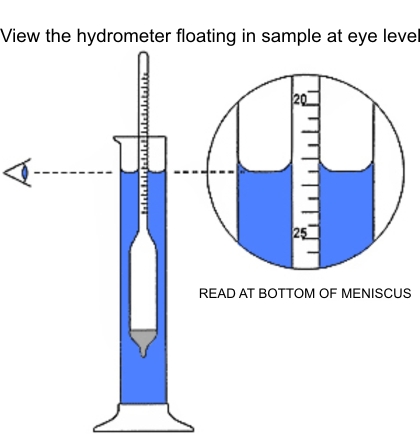
Carefully immerse a clean. dry salometer into the brine. Assure that the salometer is floating and not touching the cylinder walls. After the salometer has stabilized. take a reading at the brine surface at eye level at the bottom of the miniscus. the concavity formed on the cylinder walls at the brine surface (see illustration).
Check new salometers by first placing them in clear water, where the reading should be nearly O°S at 60°F. Empty the cylinder, rinse it with saturated salt solution and then refill with saturated brine at 60°F. The reading should be near 100°S’and not less than 98°S.
Errors in reading a Salometer
Considerable error may result from readings made with the correct Salometer at the correct temperature if proper procedure is not used. The following suggestions will help in securing correct Salometer readings.
Temperature of the brine should be the same as specified in the brine table being used. A 60ºF Salometer will not give a correct reading at 38ºF and vice versa.
Brine should be tested only in a straight walled cylinder of clear glass, set solidly on a level surface. Any moisture that collects on the outside of the cylinder should be wiped off.
Make sure that the Salometer stem is dry, clean, and free from grease, or caked salt crystals, and that the Salometer does not touch the sides of the cylinder when readings are taken.
Check new Salometers by placing them first in clear water, when the reading should be 0ºS at 60ºF. Empty the cylinder, rinse with a saturated salt solution, then refill with saturated brine at 60ºF. Salometer should read 100ºS.
Care must be taken to read the scale marking at the actual surface of the brine when the Salometer has come to rest. This brine surface is not level, as brine tends to rise along the sides of the cylinder and along the stem of the Salometer, forming a concave surface known as a meniscus. For a correct reading, bring the eye to a point level with the bottom of the meniscus.
Explanation of Hydrometer Scales
Density is defined as weight per unit volume (pounds per gallon, grams per milliliter, pounds per cubic foot, etc.) Specific gravity of liquids and solids is the density compared with that of water at 4ºC.
Density of liquids is determined:
(1) by weighing a known volume, or weighing equal volumes of water and the liquid and comparing (pycnometer);
(2) by determining the loss in weight of a plummet of known volume weighed in air and in the liquid, or by comparing the weight of a plummet of unknown volume weighed in water (at 4ºC) and in the liquid
(Westphal balance); or
(3) by means of hydrometers, weighted glass floats which sink in the liquid to a depth dependent on the density, which is read at the liquid line on a calibrated stem extending above the liquid.
Hydrometers are calibrated
(1) in terms of specific gravity of liquid at 60ºF compared with water at 60ºF (called Sp. Gr. 60º / 60ºF);
(2) in percentage of a substance in a solution or mixture; or
(3) in arbitrary divisions, such as degrees Baumé (Be.) degrees Twaddell (Tw.), degrees Salometer (S.).
For Liquids Heavier than Water
| Degrees Salometer | by far the most common of all the hydrometer scales used for testing brines. The scale indicates directly the percent saturation of the brine, reading 0ºS in pure water, and 100ºS in fully saturated brine. Since saturated brine contains 26.395% salt by weight, each Salometer degree represents 0.26395% salt. |
| Degree Barkometer | commonly used for testing density of tanning liquors. ºBk = 1000 (Sp. Gr. – 1.000) |
| Degrees Twaddell | a scale similar to the Barkometer scale, and widely used in England. ºTw = 200 (Sp. Gr. -1.000) |
| Degrees Baumé | an arbitrary scale originally intended to indicate % salt in brine. ºBe = 145 145 Sp. Gr. |
| Degrees Brix | (Balling) used in the sugar industries; each degree Brix represents percent sugar (sucrose). |
| Digital Stem Thermometers | Therm-100_ButtonCellBattery.pdf |
| 895,821,801,850,RT8002,811CE,308’s, Bench Hygrometers | Therm-101-Carbon-Zinc Battery.pdf |
| Dataloggers,FridgeTags,LogTags, IR Thermometers, Tachometers | Therm-103-Lithium-Manganese Button Cell Battery.pdf |
| THERMOMETER LIQUIDS | THERMOMETER LIQUIDS.pdf |
| Bottles Filled With BioSafe Medium | Therm-102-Dynalene BioGlycohl.pdf |
| Mercury (Hg) Filled Thermometers | Therm-104-Quecksilber.pdf Therm-104-Quecksilber-French.pdf |
| Blue Spirit Filled Thermometers | Therm-105_Isoamyl benzoate for synthesis.pdf |
| Therm-106_Petroluem62.pdf | |
| Therm-107_Pentane.pdf | |
| Low Temp (-50/50°C) Spirit Filled Thermometers | Therm-108_Toluene.pdf |
| Therm-109-Pentane.pdf | |
| Therm-110_MicroScan Mineral Oil.pdf |
Includes all current products of Liquid in Glass and Digital Thermometers, Hygrometers, Hydrometers, Data Loggers. IR Thermometers and more.
Includes all current Data Loggers and Monitors.
Includes all current Vaccine, Fridge, Freezer Temperature Data Loggers and Monitors.
Featuring…
RASCHER & BETZOLD
A.S.B.C. Top Reading
Brand Glass Hydrometers
for the Brewing Industry

Thermco Instructional Video ACC821

Thermco Instructional Video ACC895

Thermco Instructional Video ACC850

Thermco Instructional Video TRED30-16R Single Probe Vaccine Data Logger